Tender are the vegetables
You may have found yourself in the frustrating situation of cooking the perfect baked potatoes. I've tried for years. I'll spare you my unsuccessful attempts, but I just tell you that I have never managed to get them the way as I wanted: crunchy outside, tender inside.
And then it happed: the revelation came on the radio, on a rainy Saturday afternoon. I was listening to a recording of an interview of Dario Bressanini on Radio Deejay, where he explained the secret for the pefect baked potatoes. The light bulb went on my head and I suddenly realized I had the answer in my hands for years: it was well described in one of Herve’ This’ books!
The scientific explanation of the week
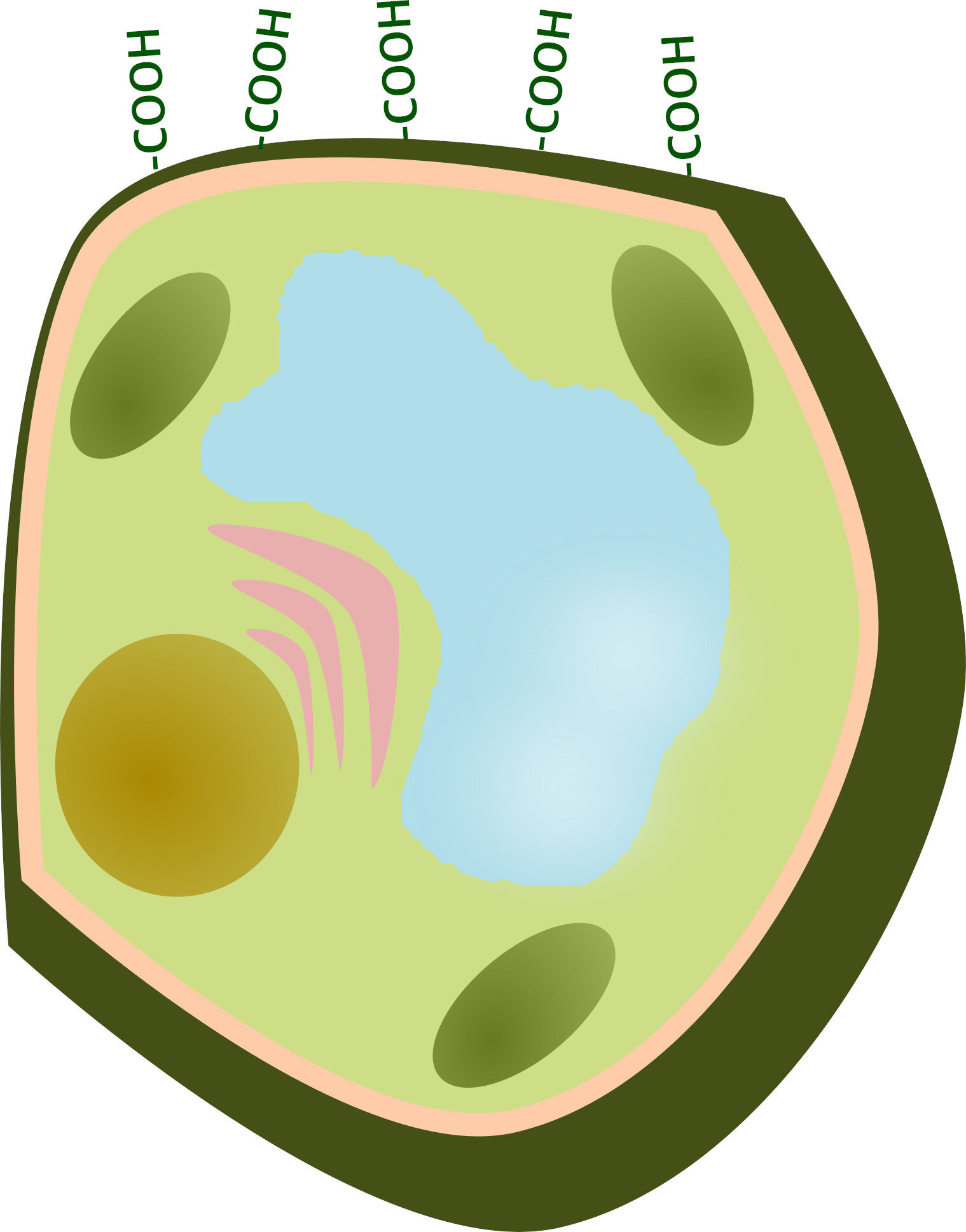
Did you miss it? :) Well, you should know that there is a trick to make vegetables more tender. That is to add sodium bicarbonate (or baking soda) to the cooking water. The walls of plant cells are made up of pectins (deja-vu? :)), among other things. Pectins have carboxylic groups, -COOH, which have an acidic character and deprotonate at basic pH at -COO-. Negative charges repel each other, causing the cell walls to open.
I had already talked about these properties of pectins when I explained their use in the preparation of jams. However, I would not have thought of using it for the preparation of vegetables. How to get tender vegetables? Just add some baking soda to the cooking water! The alkaline pH provokes the deprotonation of the carboxylic groups, the opening of the cell walls and the entry of water. On the other hand, if the cooking water is acidified (for example with vinegar or lemon juice), the -COOH groups remain protonated and the walls get closer.
The hardness of the water
So, just adding baking soda? That's all? Well, there would be one point not to be overlooked...the hardness of the water. Very hard waters are rich in calcium ions, which have two positive charges (Ca2 +). These ions are therefore able to bind two -COO- groups, acting as a bridge and bringing the walls together. In short, adding sodium bicarbonate may not be enough if the cooking water is very hard.
My 2 cents
This cooking method can be applied a little to all vegetables. However, I think it is particularly suitable for those that require long cooking, such as potatoes, carrots and legumes. You also need to pay attention to the timing, because the vegetables cook quickly with baking soda and can pulp.
And the recipe for baked potatoes? Next week, stay tuned :)